What Are You Looking For?
What Are You Looking For?
A Hefei company is deeply engaged in Europe, the United States, and Southeast Asia, providing customized solutions to address transportation pain points.
Amidst the accelerating collaboration in the global biopharmaceutical industry, the safety, compliance, and timeliness of biospecimen transportation have become crucial links in cross-border R&D and diagnosis. Hefei-based Advance International Corp. (hereinafter referred to as "Advance") leverages its precise understanding of regional market needs to expand into the international market through a series of benchmark collaborations. It has gradually established an overseas presence covering Europe, the United States, and Southeast Asia, establishing "Made in China" as a key player in the global biopharmaceutical supply chain.

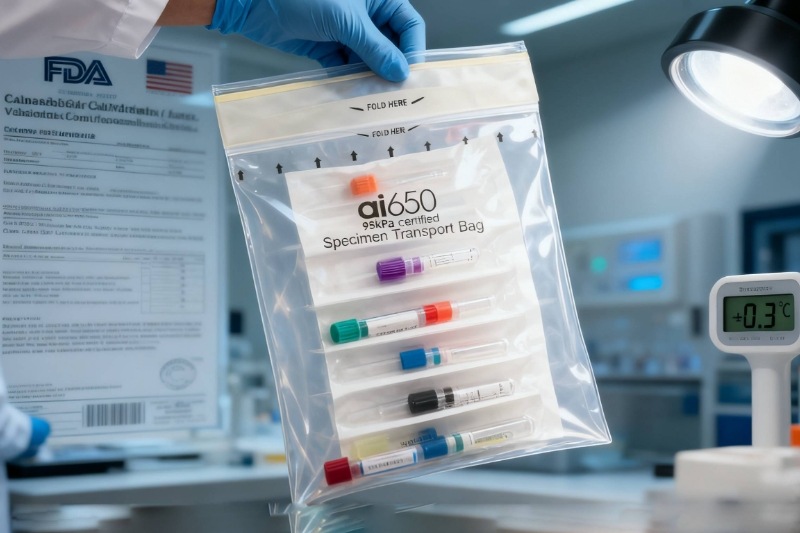
Europe is one of the regions with the most advanced medical diagnostic technology and the most stringent standards. Clinical diagnostic laboratories in Germany and France require that the transportation of tumor biopsy samples and infectious disease testing samples not only comply with the United Nations UN3373 Category B standard but also obtain EU CE certification. Furthermore, they must ensure a sample retention rate exceeding 95% within 72 hours.
To meet this need, Advance customized triple-protection transport bags for a precision oncology diagnostic laboratory in Munich, Germany. The outer layer is made of puncture-resistant medical-grade polyethylene to prevent breakage, the middle layer incorporates a moisture-absorbing buffer layer to mitigate leakage risks, and the inner layer uses a special process to control temperature fluctuations within ±1°C. Since the collaboration, the laboratory has achieved zero incidents and zero loss in sample transportation, making Advance a stable supplier. During the regular COVID-19 testing phase, Advance also supported a public health laboratory in Paris, France, helping to handle over 100,000 test samples and earning "Quality Supplier" certification.

North America, particularly the biotech cluster in California, is a global hub for innovation in mRNA vaccine development and gene therapy. Product approval requires passing 12 stringent FDA reviews, including material safety and ≥0.5kPa sealing performance testing. After two years of research and development, Advance officially received FDA certification for its transport bags in 2024, successfully entering the California market.
An mRNA vaccine development company in San Francisco faced a challenge: vaccine samples would lose effectiveness if their temperature fluctuated by more than ±0.5°C within 24 hours of being transported from the laboratory to its southern production base. Advance customized transport bags embedded with a flexible temperature buffer layer and equipped with specialized sealing zippers, ultimately controlling temperature fluctuations to within ±0.3°C, helping the company improve sample turnover by 15%. Furthermore, Advance's products entered the supply chain of a Los Angeles-based genetic sequencing company, making it the first Chinese supplier for cross-border sample transportation between the US and Canada.
The Southeast Asian biopharmaceutical industry is rapidly expanding. Singapore and Malaysia have seen investment growth exceeding 20% over the past three years. Both regions are simultaneously upgrading their cold chain infrastructure. However, specialized sample transport products suitable for gene and cell therapies are scarce and rely heavily on expensive imports. Advance seized this opportunity and took the lead in establishing a presence in the local market.
In Singapore, Advance is collaborating with a CAR-T cell therapy company. CAR-T cell samples require low-temperature storage and vibration protection. Advance leveraged the local transportation characteristics of "short-distance land transportation + port transshipment" to optimize the shock-absorbing structure of the transport bag while also using lightweight materials to reduce costs. Following this collaboration, samples were transported from Singapore to a treatment center in Kuala Lumpur, Malaysia, with cell viability consistently exceeding 98%, significantly reducing treatment costs. Furthermore, Advance participated in supporting projects at the Malaysia Biopharmaceutical Industrial Park, becoming a "preferred partner" for five R&D institutions within the park, establishing a strong foothold in emerging markets.
From responding to regulatory compliance requirements in Europe, adapting cutting-edge technologies in North America, and tapping into emerging opportunities in Southeast Asia, Advance consistently employs a "demand-driven, customized solution" approach to address the pain points of cross-border sample transportation for global biopharmaceutical clients and continuously improve the "China Solution" for biological sample transportation. For more information on our product features, regulatory certification details, or to explore customized collaboration opportunities, please visit our official website: www.aicbiologicalbag.com.